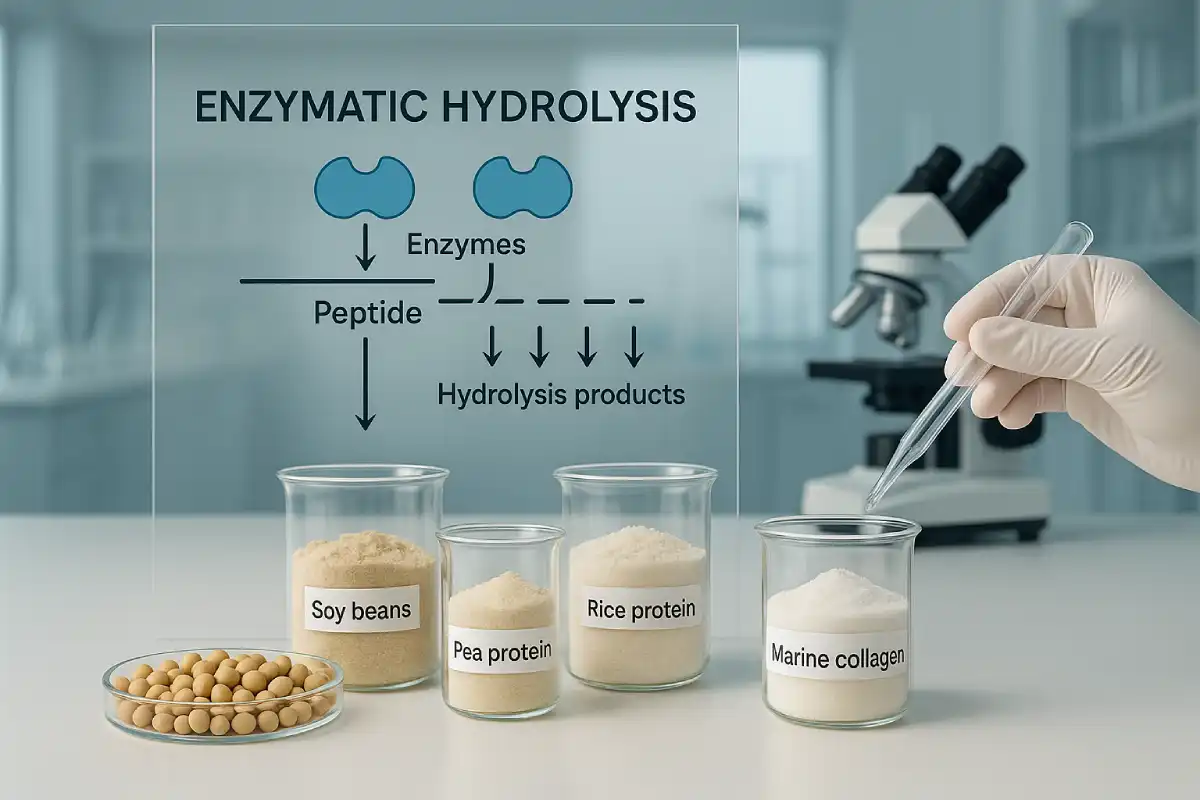
Why Strain Selection Matters in High-Efficacy Probiotics
In the competitive functional nutrition market, probiotics are no longer defined merely by colony-forming units (CFU) on a label. B2B clients—from functional food manufacturers to nutraceutical developers—are now focusing on strain-level efficacy, validated mechanisms, and industrial scalability. Choosing the right strain determines not only product performance but also the credibility and market success of the brand behind it.
BIOXMAS®, the probiotic division of EYOSON® Group, exemplifies this evolution. As a biotechnology enterprise focused on strain screening, functional validation, high-activity industrialization, and multi-dimensional applications, BIOXMAS® integrates advanced R&D and large-scale production capabilities. It has established both the world’s first all-domain ecological strain bank and the first marine probiotic strain library, housing over 10,000+ strains across human, animal, and environmental origins. This unique resource enables the precise development of next-generation probiotics with proven bioactivity and stability.

1. The Strategic Importance of Strain-Level Selection
Most commercial probiotics today are labeled at the species level—Lactobacillus acidophilus, Bifidobacterium bifidum, Lacticaseibacillus rhamnosus, and so on. However, scientific consensus emphasizes that probiotic functions are strain-specific rather than species-general [1]. For instance, while one Lactobacillus plantarum strain may promote gut barrier integrity, another may show antioxidant potential instead.
For B2B brands, this means efficacy claims, regulatory filings, and consumer outcomes depend entirely on the specific strain chosen. Strain-level selection thus defines:
- Mechanistic clarity – Understanding how the strain interacts with the host (e.g., immune modulation, SCFA production).
- Clinical credibility – Only strain-verified outcomes can be supported by scientific publications and health claims.
- Manufacturing feasibility – Each strain’s growth conditions, oxygen sensitivity, and stability profile influence production yield.
- Differentiation – Strain-verified claims build unique brand IP in a crowded market.
BIOXMAS®’s precision strain evaluation platform screens thousands of candidate strains through functional genomics, metabolomic profiling, and host-interaction assays—ensuring every strain introduced to market is not only biologically potent but also technologically scalable.
2. From Nature to Efficacy: The BIOXMAS® Strain Discovery Pipeline
BIOXMAS® leverages its All-Domain Ecological Strain Bank—a repository spanning soil, marine, plant, and human ecosystems—to identify robust, adaptive, and functional strains. This cross-environmental approach increases the probability of finding strains with unique resilience and novel bioactive pathways.
2.1 Multi-Source Exploration
- Human-Derived Strains: Selected from healthy gut microbiota for immune regulation, metabolic balance, and anti-inflammatory potential.
- Marine-Derived Strains: Cultivated from oceanic sources for oxidative stress resistance and biofilm inhibition—key for skin and gut health.
- Plant-Associated Strains: Adapted to polyphenol-rich environments, offering antioxidant synergy when formulated with botanical extracts.
2.2 Functional Screening & Mechanistic Validation
BIOXMAS® employs multi-omics tools to identify strains with measurable health effects:
- In vitro screening: Enzyme activity (β-galactosidase, bile salt hydrolase), adhesion to intestinal epithelial cells, and pathogen inhibition.
- Cellular models: Cytokine modulation, NF-κB inhibition, ROS scavenging.
- Animal and human trials: Validation of metabolic, immune, and cognitive benefits [2].
These data-driven layers ensure that only high-efficacy strains—those with reproducible, measurable effects—advance to industrial scaling.
3. Industrialization of High-Activity Probiotics
A critical bottleneck in the probiotic industry lies in maintaining cell viability and bioactivity throughout production, formulation, and storage. BIOXMAS®’s integrated manufacturing platform overcomes this through controlled fermentation, optimized lyophilization, and microencapsulation.
3.1 Controlled Fermentation and Stress Conditioning
Using proprietary bioreactor systems, BIOXMAS® tailors oxygen levels, pH, and nutrient composition to maximize CFU yield and stress resistance. Pre-conditioning strains under simulated gastrointestinal conditions enhances their survivability post-consumption—an essential factor for end-user efficacy.
3.2 Advanced Freeze-Drying and Microencapsulation
Post-fermentation, the company applies precision lyophilization combined with protective agents to preserve membrane integrity and enzyme function. Microencapsulation with food-grade polymers ensures stability in heat, moisture, and gastric acid environments—making the strains ideal for incorporation into functional foods, beverages, and dietary supplements.
3.3 Formulation Compatibility
BIOXMAS® probiotics are validated for compatibility with peptides, plant extracts, and vitamins—an advantage for partners seeking synergistic formulations such as peptide-probiotic combinations or synbiotic blends. This flexibility broadens product applications across gut, metabolic, immune, and skin health categories.
4. Evaluating High-Efficacy Probiotics: Key Technical Indicators
| Indicator | Description | Impact on Product Efficacy |
|---|---|---|
| Acid & Bile Salt Tolerance | Ability to survive gastric passage | Ensures live delivery to intestines |
| Adhesion Capacity | Affinity for intestinal epithelium | Promotes colonization and sustained benefits |
| Metabolite Profile | Production of SCFAs, GABA, antioxidants | Determines physiological functions |
| Immunomodulatory Index | Cytokine regulation (IL-10, TNF-α) | Predicts anti-inflammatory capacity |
| Stability | Resistance to temperature, humidity, oxygen | Enables shelf-life and formulation diversity |
BIOXMAS®’s proprietary Probiotic Efficacy Index (PEI) integrates these parameters into a unified score, facilitating fast and reliable strain evaluation for industrial clients.
5. Case Studies: From Lab to Market Success
Case 1: Marine-Derived Lactiplantibacillus for Skin Beauty
A Japanese nutraceutical brand partnered with BIOXMAS® to develop a marine-derived Lactiplantibacillus plantarum strain rich in antioxidant peptides. The result: a probiotic supplement clinically proven to reduce skin oxidative stress and improve elasticity after eight weeks. This product achieved 32% year-over-year growth and entered three Asian markets in under a year.
Case 2: Multi-Strain Formula for Metabolic Health
A European functional food manufacturer utilized BIOXMAS®’s All-Domain Ecological Bank to select complementary strains that modulate lipid metabolism and glucose balance. The resulting synbiotic beverage demonstrated significant improvement in serum triglyceride levels in human trials [3], leading to commercial launch across EU and North America.
Case 3: Infant Formula Application
For an infant nutrition client, BIOXMAS® identified a Bifidobacterium breve strain with strong adherence to intestinal epithelial cells and safe genomic profile. Through high-activity industrialization, the strain achieved a CFU stability of >90% after 24 months—meeting EU infant formula standards. The product has since been adopted by multiple premium formula brands.

6. Regulatory and Quality Assurance Framework
To ensure compliance with global regulatory standards, BIOXMAS® integrates traceability and quality assurance across the entire R&D-to-production chain:
- GMP-certified manufacturing with full batch traceability.
- Whole-genome sequencing (WGS) for safety validation and strain identity.
- ISO 17025 testing laboratories ensuring quantitative accuracy.
- Clinical validation compliant with EFSA and FDA guidance.
For brands seeking entry into multiple markets, BIOXMAS®’s regulatory team provides documentation support for GRAS, Novel Food, and Health Claim submissions—significantly reducing time-to-market risk.
7. Trends Shaping the Future of Probiotics
The next generation of probiotics will not rely solely on traditional gut health positioning. Instead, innovation is shifting toward precision-targeted functions and cross-disciplinary integrations.
7.1 Precision Probiotics and Omics Integration
Advances in metagenomics, transcriptomics, and metabolomics allow targeted strain design. BIOXMAS® uses these tools to identify functional gene clusters responsible for key bioactivities, such as GABA synthesis for stress relief or SCFA pathways for metabolic balance [4].
7.2 Probiotic-Peptide Synergy
An emerging trend is the combination of bioactive peptides and probiotics to enhance absorption and resilience. With EYOSON®’s peptide expertise and BIOXMAS®’s strain technology, the group enables cross-functional formulation of peptide-symbiotic systems—bridging two leading domains in functional nutrition.
7.3 Marine Biotechnology
The marine ecosystem is a vast reservoir of unexploited probiotic species with natural antioxidant and anti-inflammatory potential. BIOXMAS®’s Marine Strain Library pioneers this frontier, offering brands the opportunity to differentiate with ocean-origin probiotics validated for both gut and skin benefits.
8. Choosing the Right Probiotic Partner
For functional nutrition and healthcare brands, partnering with a probiotic manufacturer involves more than purchasing bulk powder. It’s a strategic collaboration that defines scientific integrity, scalability, and consumer trust.
When evaluating partners, prioritize those who offer:
- Comprehensive Strain Libraries – Access to diverse and verified microbial resources.
- Functional Validation – Omics-based and clinical verification of bioactivity.
- Industrial Competence – Proven capabilities in large-scale, high-activity fermentation.
- Regulatory and Formulation Support – Guidance across global compliance and product development.
- Innovation Ecosystem – The ability to co-develop next-generation functional ingredients.
BIOXMAS® and EYOSON® together embody these principles—integrating biotechnology, industrial production, and market foresight to support partners from R&D concept to global commercialization.
9. Building the Next Generation of Probiotic Brands
As the functional nutrition market matures, probiotic innovation will increasingly hinge on strain precision, mechanistic validation, and sustainable scalability. Strain selection is not merely a technical decision—it is a brand-defining strategy.
BIOXMAS®, powered by EYOSON®’s research and manufacturing ecosystem, stands at the forefront of this transformation. With 10,000+ strains in its all-domain and marine libraries, advanced omics-driven screening, and robust industrial infrastructure, it enables B2B partners to create science-based, market-ready probiotic products with measurable health benefits.
For global brands seeking differentiation and trust, the message is clear:
Strain selection matters—and the right partner makes all the difference.
Partner with BIOXMAS® for High-Efficacy Probiotics
Access 10,000+ validated probiotic strains from our All-Domain Ecological and Marine Libraries. Co-develop next-generation functional ingredients with proven bioactivity, industrial scalability, and regulatory support.
Explore Strain SolutionsFAQ
Strain-level selection ensures that your product delivers predictable, reproducible health benefits. Different strains of the same species can have vastly different bioactivities. For B2B brands, using strain-verified probiotics reduces regulatory risk and enhances consumer trust [1].
BIOXMAS® uses a multi-layered screening approach: in vitro assays, cellular models, omics-driven profiling, and animal/human trials. This ensures only industrial-ready, functionally potent strains advance to formulation.
Marine probiotics often exhibit enhanced oxidative stress resistance and unique metabolic profiles. Using strains from BIOXMAS®’s Marine Probiotic Library allows brands to differentiate products with ocean-origin functional claims.
BIOXMAS® validates strains for beverages, functional foods, powdered supplements, synbiotic or peptide-combined formulas, and temperature-sensitive formulations via microencapsulation or freeze-drying.
BIOXMAS® offers GMP-certified production, full batch traceability, whole-genome sequencing for safety, ISO 17025 validation, and guidance on GRAS, Novel Food, and Health Claim documentation.
Differentiation comes from unique, strain-verified bioactivity and innovative applications such as marine-origin probiotics, multi-strain synbiotic formulas, and peptide-probiotic synergy.
Key criteria include comprehensive strain libraries, functionally validated strains, scalable industrial production, regulatory and formulation support, and innovation ecosystem for co-development opportunities.
Yes. Examples include marine Lactiplantibacillus for skin benefits, multi-strain synbiotic beverages for metabolic health, and Bifidobacterium breve in infant formula with >90% CFU stability over 24 months.
References
- Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., … & Sanders, M. E. (2014). The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11(8), 506–514.
- Ouwehand, A. C., & Salminen, S. (2020). Probiotic functionality: Mechanisms, efficacy, and safety. Microbial Ecology in Health and Disease, 31(1), 162–170.
- Chen, L., et al. (2021). Probiotic supplementation improves lipid metabolism and insulin sensitivity: A randomized clinical trial. Nutrition Research, 92, 76–84.
- El Hage, R., et al. (2022). Omics approaches in probiotic discovery and characterization. Frontiers in Microbiology, 13, 832198.